| STIM1 Controls Neuronal Ca2+ Signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior
|
| May 7, 2014
|
| Hartmann J, Karl RM, Alexander RP, Adelsberger H, Brill MS, Rühlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, Misgeld T, Konnerth A
|
| Neuron
|
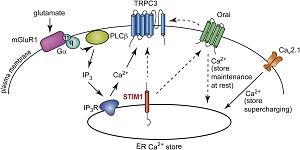 In central mammalian neurons, activation of metabotropic glutamate receptor type1 (mGluR1) evokes a complex synaptic response consisting of IP3 receptor-dependent Ca(2+) release from internal Ca(2+) stores and a slow depolarizing potential involving TRPC3 channels. It is largely unclear how mGluR1 is linked to its downstream effectors. Here, we explored the role of stromal interaction molecule 1 (STIM1) in regulating neuronal Ca(2+) signaling and mGluR1-dependent synaptic transmission. By analyzing mouse cerebellar Purkinje neurons, we demonstrate that STIM1 is an essential regulator of the Ca(2+) level in neuronal endoplasmic reticulum Ca(2+) stores. Both mGluR1-dependent synaptic potentials and IP3 receptor-dependent Ca(2+) signals are strongly attenuated in the absence of STIM1. Furthermore, the Purkinje neuron-specific deletion of Stim1 causes impairments in cerebellar motor behavior. Together, our results demonstrate that in the mammalian nervous system STIM1 is a key regulator of intracellular Ca(2+) signaling, metabotropic glutamate receptor-dependent synaptic transmission, and motor coordination. In central mammalian neurons, activation of metabotropic glutamate receptor type1 (mGluR1) evokes a complex synaptic response consisting of IP3 receptor-dependent Ca(2+) release from internal Ca(2+) stores and a slow depolarizing potential involving TRPC3 channels. It is largely unclear how mGluR1 is linked to its downstream effectors. Here, we explored the role of stromal interaction molecule 1 (STIM1) in regulating neuronal Ca(2+) signaling and mGluR1-dependent synaptic transmission. By analyzing mouse cerebellar Purkinje neurons, we demonstrate that STIM1 is an essential regulator of the Ca(2+) level in neuronal endoplasmic reticulum Ca(2+) stores. Both mGluR1-dependent synaptic potentials and IP3 receptor-dependent Ca(2+) signals are strongly attenuated in the absence of STIM1. Furthermore, the Purkinje neuron-specific deletion of Stim1 causes impairments in cerebellar motor behavior. Together, our results demonstrate that in the mammalian nervous system STIM1 is a key regulator of intracellular Ca(2+) signaling, metabotropic glutamate receptor-dependent synaptic transmission, and motor coordination.
|
 In central mammalian neurons, activation of metabotropic glutamate receptor type1 (mGluR1) evokes a complex synaptic response consisting of IP3 receptor-dependent Ca(2+) release from internal Ca(2+) stores and a slow depolarizing potential involving TRPC3 channels. It is largely unclear how mGluR1 is linked to its downstream effectors. Here, we explored the role of stromal interaction molecule 1 (STIM1) in regulating neuronal Ca(2+) signaling and mGluR1-dependent synaptic transmission. By analyzing mouse cerebellar Purkinje neurons, we demonstrate that STIM1 is an essential regulator of the Ca(2+) level in neuronal endoplasmic reticulum Ca(2+) stores. Both mGluR1-dependent synaptic potentials and IP3 receptor-dependent Ca(2+) signals are strongly attenuated in the absence of STIM1. Furthermore, the Purkinje neuron-specific deletion of Stim1 causes impairments in cerebellar motor behavior. Together, our results demonstrate that in the mammalian nervous system STIM1 is a key regulator of intracellular Ca(2+) signaling, metabotropic glutamate receptor-dependent synaptic transmission, and motor coordination.
In central mammalian neurons, activation of metabotropic glutamate receptor type1 (mGluR1) evokes a complex synaptic response consisting of IP3 receptor-dependent Ca(2+) release from internal Ca(2+) stores and a slow depolarizing potential involving TRPC3 channels. It is largely unclear how mGluR1 is linked to its downstream effectors. Here, we explored the role of stromal interaction molecule 1 (STIM1) in regulating neuronal Ca(2+) signaling and mGluR1-dependent synaptic transmission. By analyzing mouse cerebellar Purkinje neurons, we demonstrate that STIM1 is an essential regulator of the Ca(2+) level in neuronal endoplasmic reticulum Ca(2+) stores. Both mGluR1-dependent synaptic potentials and IP3 receptor-dependent Ca(2+) signals are strongly attenuated in the absence of STIM1. Furthermore, the Purkinje neuron-specific deletion of Stim1 causes impairments in cerebellar motor behavior. Together, our results demonstrate that in the mammalian nervous system STIM1 is a key regulator of intracellular Ca(2+) signaling, metabotropic glutamate receptor-dependent synaptic transmission, and motor coordination.